electrochemistry khan academy|class 12 chemistry electrochemistry notes : Bacolod Unit Test - Electrochemistry | Khan Academy
25 de jan. de 2023 · On 17 July 2014 Malaysia Airlines flight MH17 was brought down over eastern Ukraine. The flight was on its way from Amsterdam to Kuala Lumpur. On board were 283 passengers and 15 crew members. Among the passengers were 196 Dutch nationals. In the aftermath the government set three priorities: repatriating and identifying the victims, .
0 · which is the negative electrode
1 · what is electrochemistry used for
2 · khan academy electrolytes chemistry
3 · free practice electrochemistry exam
4 · electrochemistry problems
5 · electrochemistry class 12 khan academy
6 · class 12 chemistry khan academy
7 · class 12 chemistry electrochemistry notes
WEBJustWatch. Perfil. Ficha técnica. Comentários. Notícias. 16 - Não recomendado para menores de 16 anos. Uma família deve proteger seus filhos de fenômenos causados .
electrochemistry khan academy*******Spontaneity and redox reactions. Free energy and cell potential. Worked example: Constructing a galvanic cell using reduction potential values. Worked example: .Free Energy and Cell Potential Opens a Modal - Electrochemistry | Khan AcademyCommercial Cells - Electrochemistry | Khan Academy
Standard Reduction Potentials Opens a Modal - Electrochemistry | Khan Academy
class 12 chemistry electrochemistry notesStandard Cell Potentials - Electrochemistry | Khan AcademyUnit Test - Electrochemistry | Khan AcademyCourse: MCAT. Electrochemistry. Google Classroom. We encounter .Learn. Introduction to electrolysis. Quantitative electrolysis. Electrolysis of .Electrochemistry questions. Google Classroom. Which of the following . Comparing a voltaic cell to an electrolytic cell. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/electrolytic .
In this video, we will discuss Faraday's first law of electrolysis. Practice this concept - https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74. What happens when you add zinc to a solution of copper sulfate? Identifying the half reactions to see what got oxidized and reduced. Watch the next lesson: h.Khan Academy
This video talks about the various types of conductivities that we come across in electrochemistry. 00:00 - Introduction 1:25 - Conductance (G) 1:45 - Conductivity (k) 6:58 - Molar conductivity.
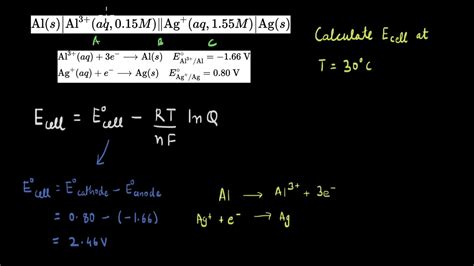
Electrochemistry questions. Google Classroom. Which of the following statements accurately describes the Nernst equation shown below? E c e l l = E c e l l o − R T n F l .
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by .Community questions. Learn AP Chemistry using videos, articles, and AP-aligned practice. Review the fundamentals of atomic structure, intermolecular forces and bonding, chemical reactions, kinetics, thermodynamics, and equilibrium.
AP Chemistry equations and constants. Electrolysis equation: I = Q t. Electrolysis variables and constants: I = current (amperes) Q = charge (coulombs) t = time (seconds) Faraday’s constant, F = 96,485 coulombs per mole = 96,485 of electrons. Learn for free about math, art, computer programming, economics, physics, chemistry, biology . So, we have two OH⁻ anions. They got their extra electrons from the Mg, so Mg has a charge of +2, so it is a cation. Thus, the second thing occurring is that we have an ionic bond . Another one that's often used is OIL RIG. And this, essentially-- oxidation is losing electrons, reduction is gaining electrons. Learn for free about math, art, computer programming, .electrochemistry khan academyLearn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, .

Comparing a voltaic cell to an electrolytic cell. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation .
electrochemistry khan academy class 12 chemistry electrochemistry notes This is because the copper is more electronegative than the zinc. The cell potential for this reaction is +1.10, since copper is being reduced and zinc is being oxidized, and if you plug this into the free energy equation for a cell ,ΔG= −n× F × EMF, you get ΔG= −2e-× (9.64 x 10^4 F) × (+1.10V) = -212 kJ. At our other electrode, the battery pulls electrons away from copper. Solid copper is oxidized; we lose two electrons to form cu 2+. So, the copper electrode loses mass over time. When we look at our problem, this is a quantitative electrolysis problem, because they're telling us what the current is: 5.0 amps.
They're giving the reaction going from zinc ions, grabbing some electrons, and becoming solid zinc. We want the other reaction. This is the reaction that we need to occur for our galvanic cell. So this reaction right over here is going to be the negative of that. So it's going to be positive 0.76 volts. Video transcript. - A concentration cell is a cell that has the same electrodes on both sides. So here we have zinc electrode on the left, and zinc electrode on the right. The only difference is the concentration. On the left side there's a .So we can write the Nernst equation once again, alright, so E, or the cell potential, is equal to the standard cell potential E zero, minus 0.0592 over n. And we essentially just change this from natural logarithm to base 10 logarithm, so this would be log of Q, log of the reaction quotient. So here is just another form of the Nernst equation.Share your videos with friends, family, and the worldSo we can write the Nernst equation once again, alright, so E, or the cell potential, is equal to the standard cell potential E zero, minus 0.0592 over n. And we essentially just change this from natural logarithm to base 10 logarithm, so this would be log of Q, log of the reaction quotient. So here is just another form of the Nernst equation.Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.Spontaneity and redox reactions. Free energy and cell potential. Worked example: Constructing a galvanic cell using reduction potential values. Worked example: Identifying the best oxidising agent using Eo (red.) values.
Course: MCAT. Electrochemistry. Google Classroom. We encounter electrochemical cells in all facets of our everyday lives from the disposable AA batteries in our remote controls and the lithium-ion batteries in our iPhones to the nerve cells strewn throughout our bodies.
Learn. Introduction to electrolysis. Quantitative electrolysis. Electrolysis of molten sodium chloride. This unit is part of the Chemistry library. Browse videos, articles, and exercises by topic.Comparing a voltaic cell to an electrolytic cell. Watch the next lesson: https://www.khanacademy.org/science/chemistry/oxidation-reduction/electrolytic-cell/. What happens when you add zinc to a solution of copper sulfate? Identifying the half reactions to see what got oxidized and reduced. Watch the next lesson: h.In this video, we will discuss Faraday's first law of electrolysis. Practice this concept - https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74.Khan AcademyElectrochemistry questions. Google Classroom. Which of the following statements accurately describes the Nernst equation shown below? E c e l l = E c e l l o − R T n F l n Q. Choose 1 answer: At equilibrium, E c e l l calculates to a value of 0 and the battery is considered dead because Q becomes K e q and K e q has a value of 0.
Funerária Pax do Brasil está situada no bairro Centro na cidade de Araguari, MG. Nosso telefone para contato: (34) 3241.. Atuamos no ramo de Funerárias com excelente .
electrochemistry khan academy|class 12 chemistry electrochemistry notes